Several types of batteries are available, each with their unique chemical compositions and properties. One of the most common types is the lead-acid battery, which is widely used in automobiles, boats, and other applications that require high power output. Another popular type is the lithium-ion battery, which is commonly found in portable electronics and electric vehicles. Lithium-ion batteries have gained widespread attention in recent years due to their high energy density and long cycle life.
Batteries play a vital role in modern society, powering a vast array of electronic devices and renewable energy storage systems. Several different types of batteries are available, each with its unique set of advantages and disadvantages, and the choice of battery chemistry will depend on the specific application.
Batteries operate through complex electrochemical reactions that occur between the anode, cathode, electrolyte, and separator. The anode and cathode facilitate oxidation and reduction reactions, respectively, while the electrolyte acts as a conductive medium for the transfer of ions between the two electrodes. The separator physically separates the anode and cathode while allowing for ion transfer. The choice of materials for each of these components can vary depending on the specific battery chemistry and application. Understanding the chemistry of batteries is crucial for developing high-performance batteries for a variety of applications.
The electrodes are the components of the battery that facilitate the electrochemical reactions that produce electrical energy. There are two types of electrodes: the anode and the cathode.
The anode is the electrode where oxidation occurs during battery operation. It is typically made from a material that can release electrons, such as graphite or lithium. The cathode, on the other hand, is the electrode where reduction occurs during battery operation. It is typically made from a material that can accept electrons, such as metal oxides like lithium cobalt oxide or lithium iron phosphate.
The electrolyte is the medium that allows for the transfer of ions between the anode and the cathode. It is typically a liquid or gel-like substance that contains ions and acts as a conductive medium. The electrolyte can be either an aqueous solution or a non-aqueous solution, depending on the type of battery.
In a lithium-ion battery, for example, the electrolyte is a non-aqueous solution that contains lithium ions. This allows for the transfer of lithium ions between the anode and the cathode during battery operation.
The separator is the component that physically separates the anode and the cathode while allowing for the transfer of ions between the two electrodes. It is typically a porous material that is placed between the anode and the cathode. The separator prevents the electrodes from meeting each other, which could cause a short circuit.
During battery operation, electrochemical reactions occur at the anode and the cathode, producing electrical energy. At the anode, oxidation occurs, causing the anode material to release electrons. These electrons then flow through an external circuit, producing an electric current.
At the cathode, reduction occurs, causing the cathode material to accept electrons. This process is facilitated by the transfer of ions from the electrolyte to the cathode. The ions then combine with the electrons from the external circuit, producing a chemical reaction that releases energy in the form of electrical energy.
Lithium-ion batteries are currently the most used type of rechargeable battery due to their high energy density, long cycle life, and low self-discharge rate. They are used in a wide range of applications, from portable electronics to electric vehicles and renewable energy storage systems.
The cathode of a lithium-ion battery is typically made from a metal oxide, such as lithium cobalt oxide (LCO), lithium manganese oxide (LMO), lithium nickel manganese cobalt oxide (NMC), or lithium iron phosphate (LFP). LCO has a high energy density but can be prone to thermal instability, while LFP has a lower energy density but is more stable at high temperatures.
The anode of a lithium-ion battery is typically made from graphite, which can intercalate lithium ions during battery operation. Other anode materials, such as silicon and tin, have higher energy densities but can experience significant volume changes during cycling, which can lead to mechanical degradation.
The electrolyte of a lithium-ion battery is typically a non-aqueous solution containing a lithium salt, such as lithium hexafluorophosphate (LiPF6), in an organic solvent, such as ethylene carbonate or dimethyl carbonate. The choice of electrolyte can have a significant impact on the performance and safety of the battery.
One of the main advantages of lithium-ion batteries is their high energy density, which allows for high power output in a small package. They also have a long cycle life, which means they can be recharged and discharged many times before reaching the end of their useful life. Additionally, they have a low self-discharge rate, which means they can hold their charge for extended periods without significant loss of capacity.
However, lithium-ion batteries also have some disadvantages. They can be prone to thermal instability, which can lead to thermal runaways and, in extreme cases, battery fires or explosions. They also require careful management to prevent overcharging and over-discharging, which can reduce their lifespan and increase the risk of safety issues.
The development of batteries has revolutionized the way we use and interact with technology. While lithium-ion batteries are widely used in electronic devices, there are other types of batteries available that have their own unique properties and applications. By examining the advantages and disadvantages of each type, we can gain a better understanding of battery technology and its potential for future innovation.
Lead-acid batteries are one of the oldest types of batteries and consist of lead plates submerged in an acidic electrolyte solution. These batteries are commonly used in cars and other motorized vehicles as starter batteries. They are relatively cheap and can produce high power output in a short amount of time. However, lead-acid batteries are heavy, have a relatively low energy density, and require regular maintenance. Additionally, the lead content makes them difficult to dispose of in an environmentally friendly way.
Nickel-metal hydride batteries (NiMH) are widely used in portable electronics and power tools because of their relatively high energy density compared to lead-acid batteries. They use a nickel oxyhydroxide cathode and a hydrogen-absorbing anode to produce electricity. NiMH batteries are also relatively inexpensive and have a long lifespan, making them a suitable alternative to lithium-ion batteries. However, they are not suitable for high-power applications and have relatively low energy density compared to lithium-ion batteries.
Solid-state batteries are a new type of battery that uses solid materials instead of liquid electrolytes to produce electricity. This technology is still in development, but it offers several advantages over traditional batteries. Solid-state batteries have a higher energy density, which means they can store more energy in a smaller package. Additionally, they are safer and more durable because they are less prone to thermal runaway and do not require liquid electrolytes. Solid-state batteries have numerous potential applications, including electric vehicles, portable electronics, and renewable energy systems.
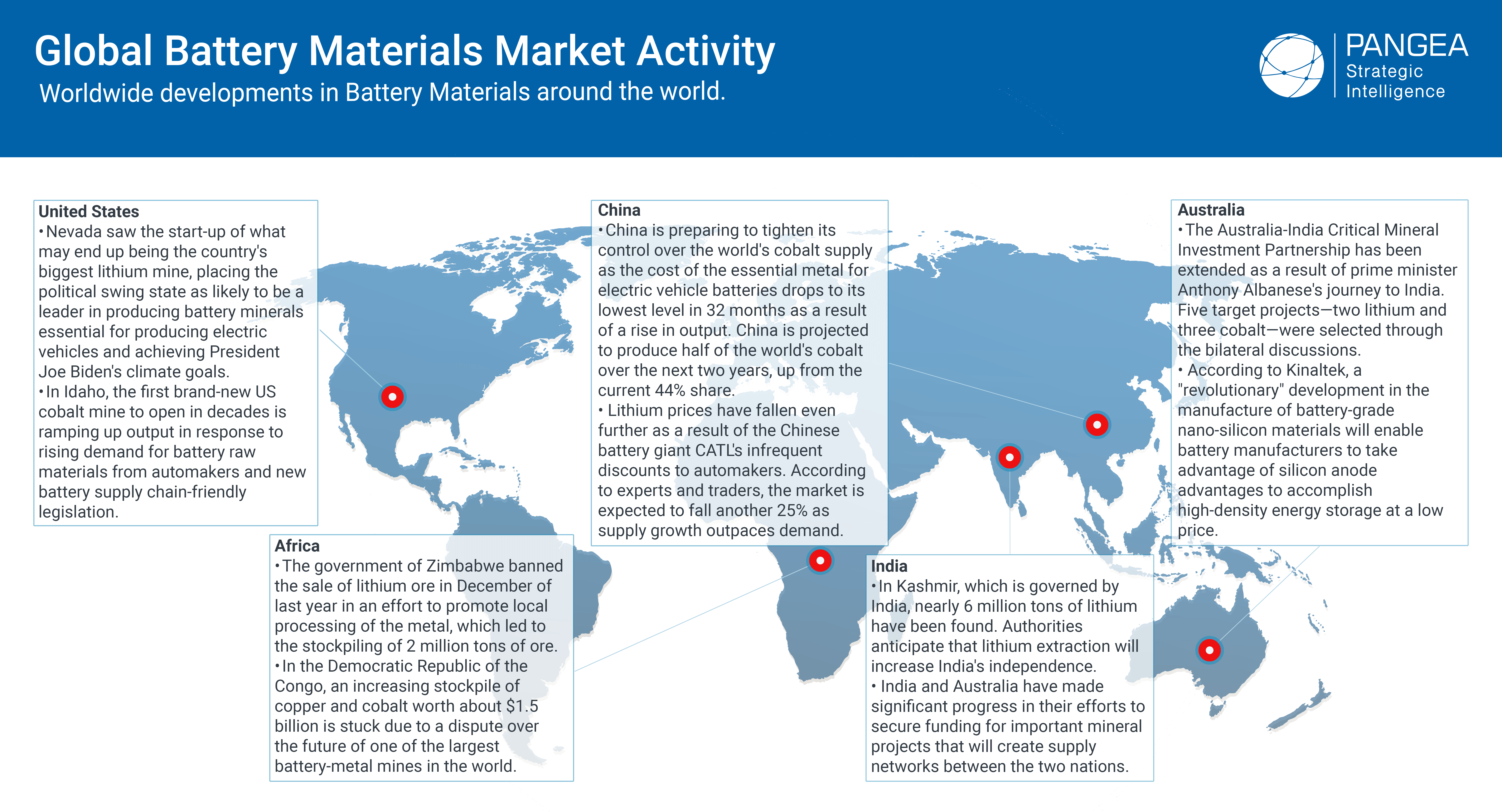
Mining battery materials is an essential step in the production of batteries, which are critical components of many modern technologies, such as electric vehicles, smartphones, and renewable energy storage systems. Battery materials are primarily mined from the earth’s crust, and the mining process has significant environmental impacts, including water and air pollution, deforestation, habitat destruction, and greenhouse gas emissions. Therefore, it is essential to consider sustainable and responsible mining practices to minimize the environmental impact of battery production.
One way to minimize the environmental impact of battery production is through battery recycling. Recycling batteries can reduce the demand for newly mined materials and decrease the amount of waste sent to landfills. Additionally, recycling can recover valuable metals such as lithium, cobalt, nickel, and manganese, which can be reused in new batteries. The recycling process involves dismantling batteries, sorting out the materials, and extracting valuable metals. The extracted materials can then be sold to battery manufacturers, creating a circular economy.
There are different types of materials mined to make batteries, including lithium, cobalt, nickel, manganese, and graphite. Lithium is the lightest metal and is the most commonly used element in rechargeable batteries. Cobalt is used to stabilize the cathode in many types of lithium-ion batteries, and it is also used in the production of hard materials such as cutting tools and jet engine parts. Nickel is used to improve the energy density and performance of batteries, and it is commonly used in electric vehicle batteries. Manganese is used to improve battery stability and safety, and it is also used in the production of stainless steel. Graphite is used as an anode material in lithium-ion batteries, and it is also used in the production of lubricants, brake linings, and carbon brushes.
The demand for more efficient, sustainable, and environmentally friendly batteries continues to increase as the world transitions to cleaner and more renewable energy sources. In response, researchers are constantly developing new materials and technologies to improve battery performance, durability, and safety.
One area of research is developing new materials for electrodes and electrolytes to improve the energy density, stability, and cycle life of batteries. For example, researchers are exploring the use of silicon, sulfur, and other high-capacity materials as anodes in lithium-ion batteries. These materials have the potential to increase the energy density of batteries, allowing for longer ranges in electric vehicles and longer run times in portable electronics.
In addition to developing new electrode materials, researchers are also working to improve the performance of electrolytes. One promising area of research is developing solid-state electrolytes that can improve battery safety, reduce the risk of fire or explosion, and increase the energy density of batteries.
Another area of research is creating more sustainable and environmentally friendly batteries. For example, researchers are exploring the use of biomaterials, such as chitin and cellulose, as a source of carbon for battery electrodes. These materials are abundant, renewable, and biodegradable, making them an attractive alternative to traditional carbon materials, which are derived from fossil fuels.
Batteries will continue to play a vital role in the growth of electric vehicles and renewable energy storage systems. As electric vehicles become more prevalent, the demand for higher energy-density batteries that can support longer ranges and faster charging times will increase. Similarly, as more renewable energy sources are integrated into the grid, there will be a need for energy storage systems that can store excess energy and deliver it when needed.
Top Six Countries with the Largest Lithium Reserves in the World
|
Bolivia – 21 million tonnes The country is thought to contain 21 million tonnes, or about one-fourth of the world’s total resource, including the Salar de Uyuni salt flat, the largest lithium deposit on Earth and visible from space. |
Argentina – 17 million tonnes With almost 17 million tonnes, Argentina has the second-largest lithium deposits in the world. These reserves, like those in the nearby countries of Bolivia and Chile, are located in vast salt flats, and their extraction requires the solar evaporation of brine pools. |
|
Chile – 9 million tonnes Chile has been successful in creating a thriving mining business for the metal, and as of 2019, it possessed the highest mine reserves in the entire globe, amounting to 8.6 million tonnes. |
United States – 6.8 million tonnes Despite having the fourth-largest lithium deposits in the world, 6.8 million tonnes, according to the US Geological Survey, the country has very little production activity. |
|
Australia – 6.3 million tonnes Despite having only the fifth-highest overall reserve with 6.3 million tonnes, Australia was by far the largest lithium producer in the world in 2019. |
China – 4.5 million tonnes With 4.5 million tonnes of total lithium reserves, China comes in sixth on the list. The nation produced 7,500 tonnes of the metal in 2019, which ranked third globally. |
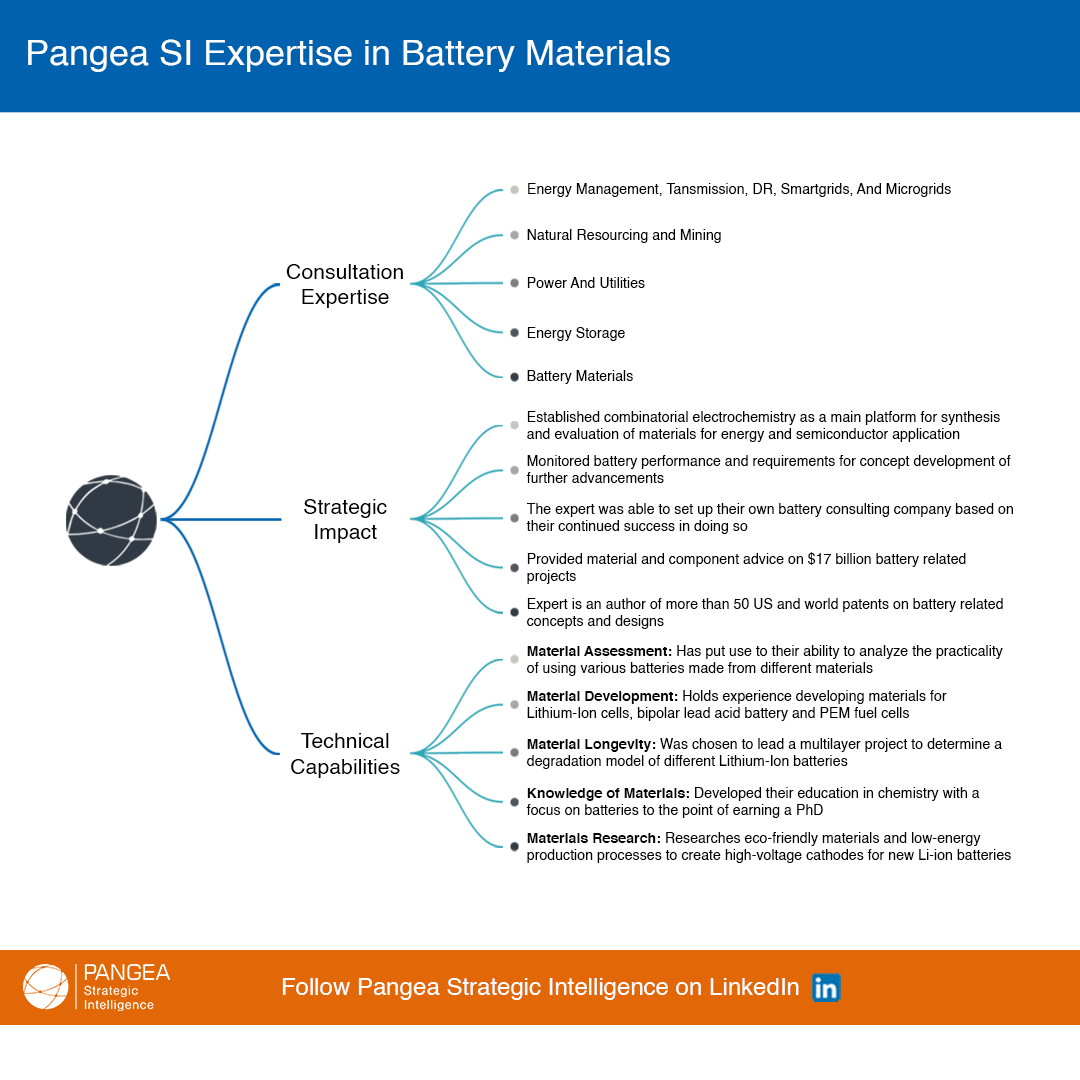
Clients notify us that they need leading insight for their project.
We engage and vet subject matter Experts matched to the request at speed.
Clients consult with their shortlisted Expert(s) and obtain forward knowledge to incorporate into their strategic plans.
Clients benefit from more refined analysis and practical guidance to achieve a quantifiable business impact.